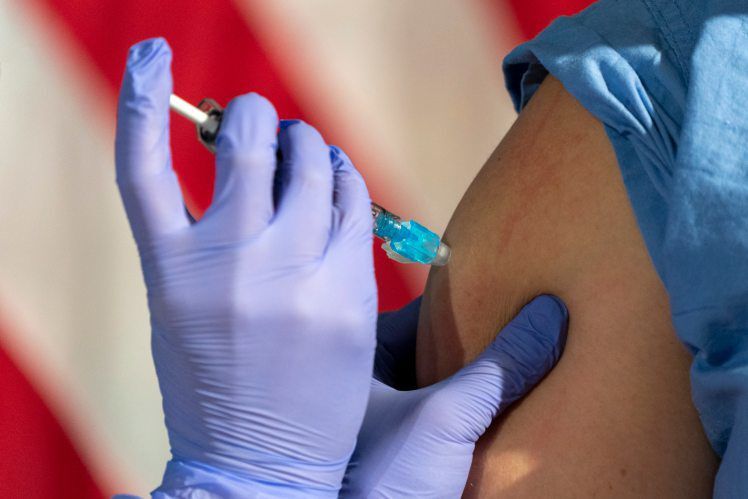
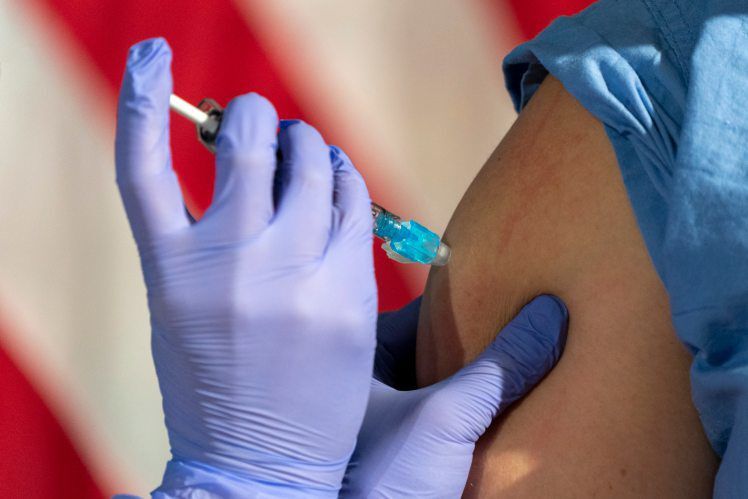
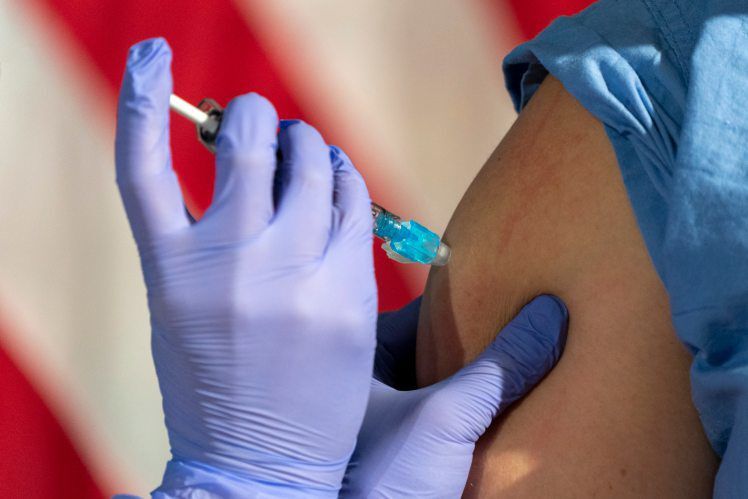
Although there’s very little data on how pregnant and nursing mothers will respond to a COVID-19 vaccine, professional organizations and individual doctors say the benefits are very likely to outweigh the risks.
Pregnant women appear to have the same chance of catching COVID-19 as everyone else. But they might fare worse if they do.
According to a November study by the U.S. Centers for Disease Control and Prevention, pregnant women are significantly more likely to be admitted to an intensive care unit, to end up on a ventilator and to die from COVID-19 than women of the same age and health status who aren’t pregnant.
So far, none of the large clinical trials of vaccines have included pregnant or nursing mothers, which is “incredibly disappointing,” said Dr. Geeta Krishna Swamy, an obstetrician/gynecologist at Duke Medical Center, who helped write the vaccine guidelines for the American College of Obstetricians and Gynecologists.
Pfizer and BioNTech, which made the first vaccine authorized for emergency use in the U.S., say they are completing tests on animals to ensure its safety before beginning trials in pregnant women early in 2021. Moderna — whose vaccine granted emergency use authorization in December — completed toxicology studies in rats, and a Food and Drug Administration review concluded it did not harm reproduction or fetal development.
Swamy, who was vaccinated in December, said data from both vaccines reassured her that unless a woman is able to isolate at home during her pregnancy, the known risks of catching COVID-19 likely outweigh the theoretical risk of vaccination. The vaccine has been shown to be safe, she said, and “is likely one of the most effective vaccines we’ve ever had.”
The risk, Swamy said, “can never be zero without data” but so far there’s nothing to suggest that pregnant women and their unborn children won’t be safe.
Dr. Jason Melillo, medical director of women’s health at OhioHealth, said he would have no qualms recommending the vaccine to a pregnant woman.
“Pregnant women can’t get COVID-19 from this vaccine,” he said. “That’s impossible.”
Most people won’t have access to the vaccine for months, to allow for more production, so likely there will be more data by the time they have access to a shot.
“For a lot of patients, if they are further along in the pregnancy and they don’t really have risk factors you will be delivered before it’s your turn in line to get the vaccine, so it will become a nonissue for you,” said Dr. Amol Arora, medical director for Women’s Health at Mount Carmel Health System.
But a large percentage of frontline health care workers are women of childbearing age and are confronting this decision now, as the first people to receive the vaccine.
“I personally feel comfortable recommending to those women that your risk benefit balance suggests you should get vaccinated,” Swamy said. “If a woman says I don’t want to get vaccinated, I think that’s absolutely, positively her choice, just like it’s her choice to get vaccinated.”
Swamy said she has even less concern about nursing mothers getting vaccinated. For the vaccine to harm a breastfed baby, she said, it would have to go from an arm into breast tissue and then into breast milk and then be digested by the baby.
“That’s a big task we’re asking that vaccine to be able to do,” she said.
The Academy of Breastfeeding Medicine shares her comfort with the COVID-19 vaccine for breastfeeding women, writing in its new guidelines that a vaccine “would be unlikely to have any biological effects” on a breastfed infant.
A woman who is vaccinated during pregnancy is likely to pass that protection on to her unborn child, with protection probably lasting some months after birth, Swamy said. Supplementing with breastmilk probably provides even longer-lasting antibodies.
Pregnant women are encouraged to get other vaccines, such as the flu shot. The only vaccine that is not recommended for pregnant women is a live virus vaccine such as the measles, mumps and rubella vaccine.
“There are lots of vaccines that we give to women in pregnancy that have been shown to be safe and this vaccine is not likely to be any different, so it’s not that we anticipate any risk for pregnant women,” said Dr. Jonathan Schaffir, an obstetrician/gynecologist at Ohio State University Wexner Medical Center.
Both COVID-19 vaccines showed similar safety and more than 94% effectiveness in large clinical trials that intentionally excluded pregnant women, although 23 women in the Pfizer-BioNTech trial and 13 in Moderna’s became pregnant during the trial. Those groups were too small to judge safety or effectiveness, and anyone found to be pregnant after the first shot did not receive the second.
The guidelines from the obstetricians and gynecologists group conclude that in the absence of specific data, a pregnant woman should make an individual decision about whether to get vaccinated.
“ACOG recommends that COVID-19 vaccines should not be withheld from pregnant individuals,” the guidelines say.
Lactating women also should be given access to the vaccine, according to the group.
Pregnant women, the guidelines suggest, should base their decision in part on how much virus is circulating in their community, as well as the risks from COVID-19. A conversation with a clinician might be helpful, according to the guidelines, “but it should not be required prior to vaccination, as this may cause unnecessary barriers to access.”
“In all likelihood it’s likely to be safe and I would recommend it,” Schaffir said.